临床需求为本
技术创新为优
同筑患者福祉

关于我们
药欣生物成立于2016年,是一家以“高端制剂研发”为核心的创新型制药企业,产品进入临床阶段。核心团队由美国知名药企科学家、专家顾问组成,具有丰富的全球商业价值判断与渠道资源整合能力,致力于风险易控、前景可期的差异化“创新药+改良型创新药”研发与产业化。公司秉持“临床需求为本,技术创新为优”的理念,凭借在难溶性药物口服制剂开发领域的技术优势,利用科研转化、知识产权、注册法规为一体的“中美跨境创新药开发平台”,开发国际Best-in-Class新药,实现技术理念与科研成果之间的转化,同筑患者福祉,共见健康未来。



我们的业务
药欣生物专注于以解决临床需求,提高患者预后为向导的新药开发。我们围绕临床需求解决关键问题,形成了先进的制剂技术和平台。基于这些具有显著竞争优势的关键平台技术,我们在新药和制剂改良开发方面形成了独特的产品布局,面向全球市场,开发符合FDA标准的创新药和改良型创新药,同时合作或引进具有潜力的CMC产品开发项目。目前,公司重点致力于肿瘤、神经系统、免疫系统疾病及过敏等领域。
新剂型新药开发
利用先进的制剂技术平台和专利制剂技术,开展特色高难改良型新药的开发,提高目标药物的生物利用度和安全性,改善患者用药依从性
新适应症新药开发
依托药欣生物国际化的科学顾问和BD团队,发挥公司科学转化项目评估体系优势,开发未满足临床需求的药品。
技术平台
药欣生物技术优势


独特的科学转化项目评估体系和策略结合国际领先的药物制剂开发平台技术,推动以临床价值为导向,开发拥有高度差异化和全球竞争力的创新药和改良型新药
-
特色创新制剂
基于物理药剂学新一代无定形固体分散体技术技术(SpraySol),以独特的配方和工艺可对多组分体系进行喷雾干燥制备固体分散体,达到物化稳定性优异的效果,对BCS 2/4分类的化合物均可有效提升口服生物利用度,对分子胶/偶联小分子药物开发(如PROTAC等)同样适用。
该创新制剂平台技术拥有完善的专利保护以及特殊配方库和特殊工艺Know-how,制备工艺稳定,可实现产业化,已有多个在研药物进入临床阶段。
-
控释制剂
优化药物释放过程,提高药物有效性及安全性,提高患者依从性。
药欣生物在控释制剂开发方面具有丰富的经验。
-
复方制剂
两种或多种活性药物成分的复方制剂,优化其药代动力学特性,可增强药效和安全性、改善患者依从性,并降低成本。
药欣生物具有开发复方制剂的专业技术。
-
新型药物递释系统
药欣生物探索搭建了多个新型药物递送技术,如多肽口服递送、siRNA药物多途径递送等。
此类新型药物递送系统可通过独特的体内分布方式,经口服、肠胃外、口腔、舌下、鼻内、局部或透皮等途径给药,优化药效、提高安全性、改善患者依从性。
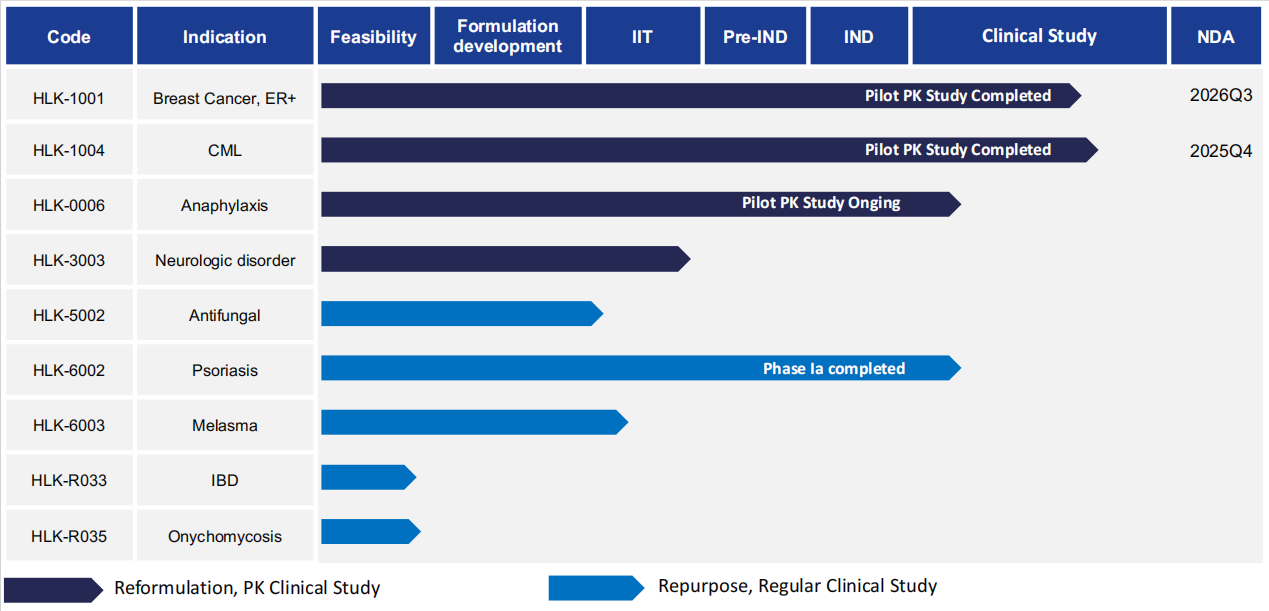
产品管线
研发产品管线
依托自身先进制剂技术平台,药欣生物已针对临床需求建立了涵盖多个治疗领域的产品管线。公司看重对现有药物进行新适应症开发,并通过505(b)(2)申报途径进行相关新药开发。
商业模式
创新,让灵感变成新药
面向包括中国及亚太地区的国际市场,开发符合中美药品监管标准的505(b)(2)新药或改良型新药。
技术授权转让产品,涵盖心血管疾病、肿瘤、免疫系统疾病及过敏等领域。
面向亚太及全球市场,合作或引进具有潜力的CMC产品开发项目。

* = 必填
联系我们
我们欢迎各方创新思维来一同应对药物开发挑战。我们愿与您一起,共创未来。如有合作意向,欢迎垂询。
HLK Pharmacin, LLC
470 James Street
Suite 007
New Haven, CT 06513 USA
203-267-3309












